Modified Albumin improves Clinical Hypoalbuminemia
Abstract:
Hypoalbuminemia has been recognized as a marker of increased mortality and morbidity since 1970. The mechanism of the worsened medical outcomes has never been elucidated. None of the current treatments of hypoalbuminemia have been successful in correcting the low albumin levels.
The application of this novel albumin product for the treatment of hypoalbuminemia has shown an increase in the serum albumin levels in 24 consecutive applications in 24 of 24 pediatric treatments in 22 patients. Although an improved clinical status was suggested; this post treatment chart review could not document other beneficial changes. No complications occurred.
This improvement in serum albumin levels has never before been accomplished. Further study continues but wider use and more complex clinical studies are needed to determine the role for the correction of hypoalbuminemia in medical treatment.
Article:
Hypoalbuminemia has been recognized as a factor associated with an increased mortality and morbidity since 1970. Multiple studies have confirmed this finding; however, no therapy has been found to treat the pathophysiology of hypoalbuminemia. The increased mortality and morbidity estimates are made by comparing normal serum albumin level patients versus low albumin patients. No studies show a benefit of albumin infusion or correction of the low serum albumin levels.
Albumin production has been measured and studied extensively; but the etiology of the cause of hypoalbuminemia remains obscure. The comprehensive Cochran Studies on the use of albumin have reviewed huge numbers of scientific studies and clinical applications and find only a small benefit of albumin treatment.
The novel albumin product is named The Norberg Solution (TNS). It is a compounded product of 5% human serum albumin and 2% hydrolyzed amino acids in Normal saline according to the guidelines expressed in USA legislation DQSA2013. This law allows the compounding of FDA approved drugs that are known to be compatible to be prescribed by the physician and compounded by the pharmacist. This product has USPO Number 7696176.
Study design:
A Phase One Trial: Case Series of Clinical Application.
All data was obtained via review of the electronic medical record.
This review did not allow analysis beyond the measurement of Albumin levels obtained during the standard medical care plan.
No patient received any additional testing or medical treatment during this care period beyond routine care and testing.
Patients were selected primarily by low serum albumin levels, any additional factors of edema and hypoperfusion as contributing factors could not be measured.
No patient was excluded for any reason -. This resulted in a very heterogeneous group of patients.
A standardized dosage of 15 Milliliters of the TNS was administered over 1-2 hours per nursing care every 12 hours for a total of three doses. (A total of 2.25 grams of albumin per kilogram body weight.) All treatments, medications, feedings and fluids were continued unmodified. Standard nursing monitoring and charting was continued.
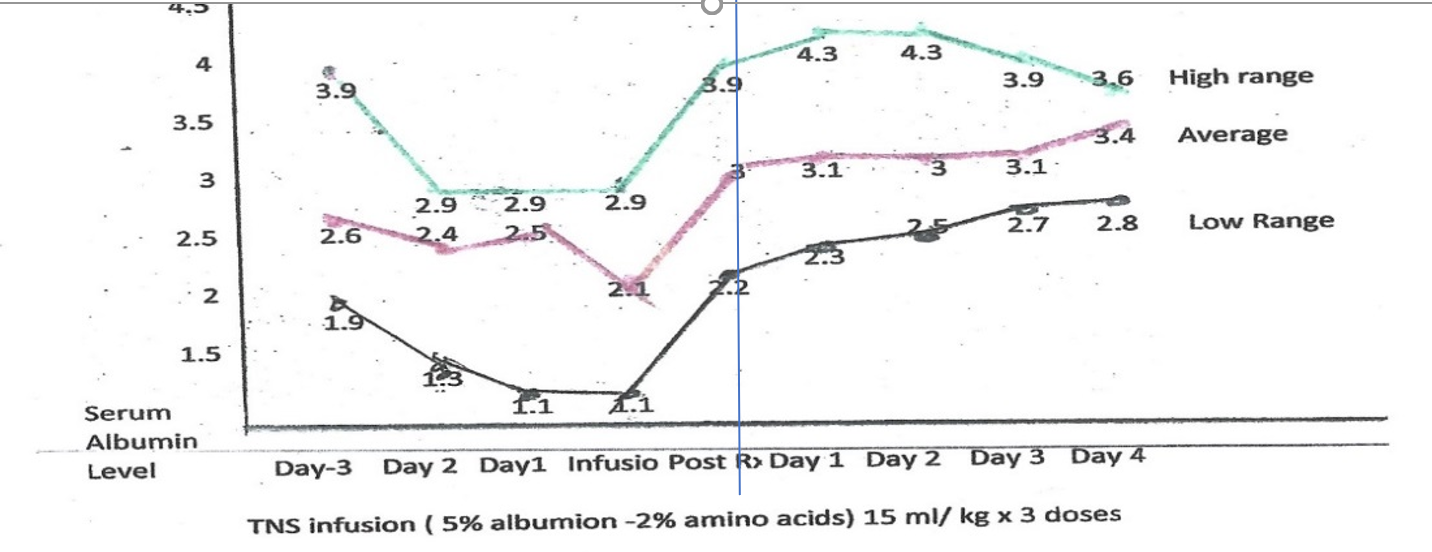
Table 1: Heterogenicity of Patients Albumin levels were measured only as deemed clinically needed.
Albumin Levels Pre and Post Albumin Administration
Number Diagnosis -3Day-2 Day -1 day Pre Post 1 Day 2 Day 3 day 4 day Change
1. Juvenile Dermatomyositis 2 2.4 2.2 2.2 3.4 2.9 3.0 2.8 2.8 1.2
2. Cholera GI Bleed 3 1.4 1.1 1.1 2.4 1.3
3. Fundoplication 2.5 2.2 2.2 2.6 2.7 3.0 1.3
4. Depressed skull Fracture 3.9 2.9 2.2 2,7 3.2 3.3 3.4 0.5
5. Juvenile Dermatomyositis 2.5 3.3 3.1 3.0 0.8
6. Juvenile Dermatomyositis 2.6 2.6 2.6 2.4 3.3 3.6 3.3 3.2 3.1 0.9
7. Respiratory Distress-Hypoxia 2.2 1.8 2.3 3.6 2.9 2.8 2.6 1.3
8. Urinary Tract Infection 2.4 2.8 2.6 2.1 3.1 1.0
9. Pleural Effusion RDS 2.0 2.0 2.5 0.5
10. Influenza A-Parainfluenza 1.9 2.2 2.0 2.0 2.5 3.0 2.9 2,7 0.5
11. Pneumonia Pleural Effusion 1.3 1.2 2.0 2.5 2.3 3.4 0.5
12. Pyelonephritis 2.3 2.8 3.4 3.7 3.9 3.8 0.5
13. Pneumonia 2.5 2.4 1.9 2.1 2.6 2.6 2.9 2.8 2.6 0.5
14. Influenza B Pneumonia 1.5 2.7 3.0 3.5 3.2 3.5 1.2
15. Mycoplasma Multiorgan Failure 2.1 2.3 2.3 3.0 3.2 2.9 2.9 2.9 0.7
16. Aspiration Pneumonia 2.2 2.1 1.9 2.4 2.6 2.5 0.5
17. Lap colostomy 3.4 1.9 2.1 3.1 2.7 2.8 3.6 1.0
18. Small bowel obstruction 3.8 2.4 2.4 2.9 3.9 3.5 3.7 3.6 1.0
19. Pneumonia & Thoroscopy 1.7 2.5 2.6 3.3 3.1 2.9 0.8
20. Pneumonia 2.9 2.5 2.6 3.9 4.3 4.3 1.3
21. Acute respiratory failure 2.1 2.0 2.3 3.0 2.7 2.9 3.3 0.7
22. Diabetes Insipidus 2.3 2.3 2.5 2.5 3.3 3.1 3.0 2.9 2.8
23. Pleural Effusion Thoracostomy 3.1 2.5 2.1 2.2 2.7 0.5
24. Fundoplication 2.3 2.3 2.5 2.5 3.3 3.1 3.0 2.9 2.8 0.8
Table 2 Graph of Albumin Values: Pre and post treatment albumin levels
Table 3. Diagnostic grouping demonstrated consistency despite differing Diagnostic catagories
Discussion:
In all cases the increase in the measured albumin levels was directly related to the TNS infusion. The average increase was 0.75 grams%. This increase was maintained for three days. In no case did the albumin levels decrease. The standard time to total body equilibration of albumin is 2 hours. These patients did not demonstrate this drug falloff rate.
No patient had any problems documented that related to TNS during or after the infusion.
Other favorable or beneficial effects were insufficiently documented in the Electronic Health Record (RHR) thereby precluding inclusion or comments in this report.
Conclusion:
This is the first report to document an intervention that results in an increase in the serum albumin levels that is maintained.
TNS is a modified Albumin-Hydrolyzed Amino Acid Compound never before available for medical therapy. The known safety of these FDA approved products permitted clinical treatment for each patient under current USA regulations.
Further studies are needed to document the effect of treatment benefit on the morbidity and mortality and to define the mechanism of benefit.
SOURCES:
Kim s, McClave SA, Martindale RG, Miller KB, Hurt RT, Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the relationship? Am Surg 2017 Nov 1,83(11) : 1220-1227
Columbo J, Codazzi D, You are Hypoalbuminemic… Ped Crit Car54e Med may 2016 Vol 17 pp430
Leite HP, Rodrigues da Siva AV, de Oliveria Iglesias S, et al: Serum albumin is an independent predictor of clinical outcomes in critically ill children. Ped Crit Care Med 2016; 17 e50-57
Wilkes MM, Navickis RJ, Patient survival after human albumin administration- meta-analysis-a meta- analysis of random controlled trials. Ann intern Med 2001 135(3); 599-610
Boldt J, Schollhorn T, Mater J, Piper S, Suttner S, The value of an albumin based intravascular volume replacement strategy in elderly patients major abdominal surgery. 2006 Anesth Analg 103(1) 191-`99
Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri S, Preoperative serum albumin level as a predictor of operative mortality and morbidity; National VA Surgical Risk Study. Arch Surg 1999: 134(1) ;36-42
Cavalho JRE, Vedelho M, New Insights About Albumin and Liver Disease. 2018 Annal of Hepatology 17(4) 547-560
Vincent JL, Relevance of Albumin in Modern Critical Care Medicine. Best Pract Res Clin Anaethesiol 2009 Jun. 23(2): 183-191
Roberts I, Blackkhall K, Alderson P, Bunn F, Scheirhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients Cochrane Database Syst Rev 2011Nov 9 CD001208
Hahn RG, Zdolsek M, Hasselgren E, Zdolsek J, Bjorne H, Fluid Volume Kinetics of 20% Albumin. R J Pharmacol2019 1-9
Leito HP, Rodriguez de Silva AV,de Olivera Igelesias SB, et al: Serum Albumin is an Independent Perdictor of Clinical Outcomes in Critically Ill Children. Ped Crit Care 2016 ; 17:e50-57
Levitt D, Levitt M, Human serum albumin homeostasis: a new look at the roles of synthesis catabolism, renal and gastrointestinal secretion and the clinical value of serum albumin measurements. Dovepress vol. 2016; pgs. 229-255 Jul 2016
Vincent jl, Russell JA, Jacob m, et al; Albumin administration in the acutely ill; What is new and what next? Crit Care 2014 18:231
Belousov A. Concept to the problems of transfusion of albumin. BJSTR 2019vol. 00318618
Appelgren KN, Rombeau JL, Twomney PL, Miller RA. Comparison of nutritional indices and outcomes in critically ill patients. Crit Care Med 1982:10:305-7
Bradley JA, Cunningham KJ, Jackson VJ, Hamilton DNH, Leadingham IM. Serum protein levels in critically ill surgical patients. Intensive Care Med 1981; 7:291-5
Foley EF, Borlase BC, Dzik WH, Bistrian BR, Bennotti PH, Albumin supplementation in the critically ill :a prospective randomized trial. Arch Surg1990. 125: 739-42
Stockwell Ma, Scot A, Day a, Riley B, Soni N, Colloid solutions in the critically ill . A randomized comparison of albumin and polygeline. 2. Serum albumin concentration and the incidence of pulmonary oedema and acute renal failure. Ana56esthesia 1992; 47: 7-9
Stockwell MA< Soni N, Riley B, Colloid solutions in the critically ill. A randomized comparison of albumin and polygeline. 1. Outcome and duration of stay in the intensive care unit. Anaesthesia 1992; 47: 3-6
Goldwasser P, Feldman J, Association of serum albumin and mortality risk. J Clin Epidemiol 1997; 50:693-703
Klonoff-Cohen H, Barrett-Connor EI, Edelstein SL, Albumin levels as a predictor of mortality in the elderly. J Clin Epidemiol 1992; 45:207-12
Reinhardt GF, Myscofski JW, Wilkins DB, Dobrin PB, Mangan RT, Stannard RT, Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. Parent Enter Nutr 1980; 4: 357-9